
The popularity of dairy-free, plant-based milk alternatives is rising. These dairy-free options, such as almond milk, soy milk, and oat milk, offer a vegan-friendly and lactose-free alternative to traditional cow's milk.
Recognizing the need for clear and informative labeling of these products, the U.S. Food and Drug Administration (FDA) issued guidance for identifying (naming) the products on the labels and for voluntary nutrient statements. Below are the key takeaways from this guidance.
How Should I Name a Plant-Based Milk Alternative?
- Standard of Identity: The FDA has not established a standard of identity for plant-based milk alternatives, so the regulations provide greater flexibility in the requirements for naming and labeling these products than for milk which is a standardized food.
- Common or Usual Names: For labeling, the name of a plant-based milk alternative product may be a common or usual name for the product established by common usage. These names often include the term "milk," e.g., "soy milk", or "almond milk." Names that include "beverage" or "drink" along with the plant source are also used e.g., “soy beverage” and “almond drink.”
- Whether to include "Milk" in the Product Name: FDA does not require the names of plant-based milk alternative products to include "milk.” For example, "beverage," or "drink” may be used in lieu of “milk” e.g., “soy drink.”
- Qualifying the Plant Source: The FDA recommends not using "plant-based milk" as the sole name for milk alternative products. When naming these products, it's important to qualify the term "milk," "beverage," or "drink" with the plant source (e.g., oat milk, almond drink, soy beverage, etc.). If a plant-based milk alternative product contains a blend of different plant sources, all the plant sources should be included in the product name with the predominant plant source listed first.
- "Imitation Milk" Labeling: FDA defines an imitation food as a “food that is a substitute for and resembles another food but is nutritionally inferior to that food.” Although not all plant-based milk alternatives meet FDA’s definition of "imitation food," FDA exercises enforcement discretion in allowing such products to be labeled as “imitation milk” because it is commonly understood by the public that plant-based milk alternatives are not milk.
- "Dairy-Free" or "Non-Dairy" Labels: Though not required, FDA encourages labeling plant-based alternative products as "dairy-free" or "non-dairy" to inform consumers that the products are not derived from animals. Whether the product name includes “dairy-free” or “non-dairy”, it should always indicate the plant source e.g., “non-dairy soy milk.”
What are the Recommendations for Voluntary Nutrient Statements?
The FDA recommends labeling plant-based milk alternative products with voluntary statements indicating the nutritional differences between plant-based milk alternatives and dairy milk.
- Content of Voluntary Nutrient Statements: A voluntary nutrient statement describes how the plant-based milk alternative's nutrient levels differ from those in dairy milk. To avoid underconsumption of certain nutrients otherwise provided by milk (calcium, protein, vitamin A, vitamin D, magnesium, phosphorous, potassium, riboflavin, or vitamin B12), FDA recommends including a voluntary statement such as:
Contains lower amounts of [nutrient name(s)] than milk
- Determining Nutrient Differences: Manufacturers who choose to include voluntary nutrient statements on their labels should compare the nutrient composition of their plant-based milk alternative to the nutrient values for dairy milk in the table below:
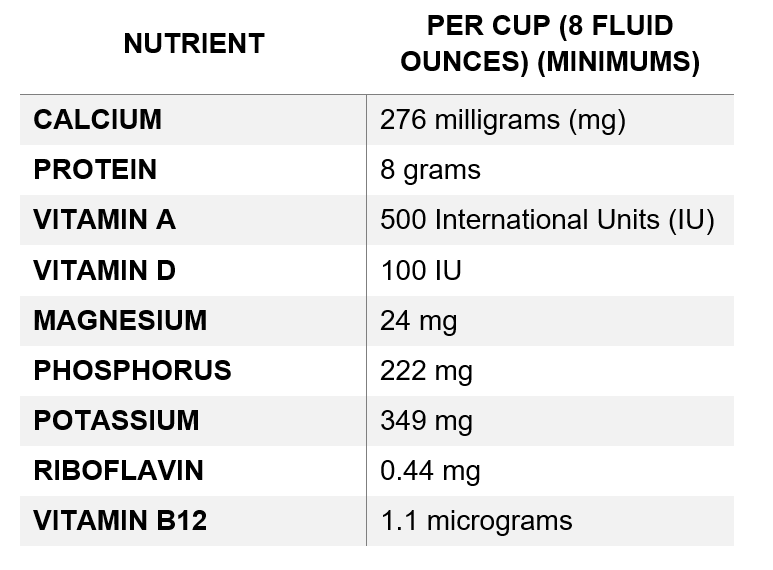
- Placement on Labels: If included, the FDA recommends that a voluntary nutrient statement be placed on the principal display panel (PDP) near and visually connected to the product name if space allows. If there is not sufficient space to accommodate this, FDA recommends placing a symbol (e.g., “*”) next to the product name that directs consumers to the statement at another location on the PDP.
The FDA's recommendations aim to ensure that consumers have clear and accurate information about the plant-based milk alternative products before purchasing them. These recommendations emphasize the need for transparent naming and labeling practices and encourage voluntary nutrient statements to help consumers understand the nutritional differences between plant-based milk alternatives and dairy milk.
Of course, FDA also has requirements for how these products must be labeled with respect to the nutrition facts chart, ingredients list, quantity of contents and other statements. We at FDA Specialist can help you ensure that the labels of your plant-based milk alternative products are compliant with FDA regulations. Contact us today for assistance!
